Jasmone
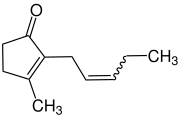
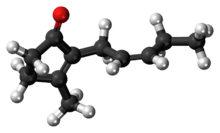
Jasmone is an organic compound, which is a volatile portion of the oil from jasmine flowers. It is a colorless to pale yellow liquid. Jasmone can exist in two isomeric forms with differing geometry around the pentenyl double bond, cis-jasmone and trans-jasmone. The natural extract contains only the cis form, while synthetic material is often a mixture of both, with the cis form predominating. Both forms have similar odors and chemical properties. Its structure was deduced by Lavoslav Ružička.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-methyl-2-[(2Z)-pent-2-en-1-yl]cyclopent-2-en-1-one | |
| Other names
cis-jasmone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H16O |
| Molar mass | 164.246 g/mol |
| Appearance | colorless to pale yellow liquid |
| Density | 0.94 g/mL, liquid |
| Melting point | 203 to 205 °C (397 to 401 °F; 476 to 478 K) |
| Boiling point | 146 °C (295 °F; 419 K) at 27 mm Hg |
Solubility in water |
in water |
| Related compounds | |
Related compounds |
jasmonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Jasmone is produced by some plants by the metabolism of jasmonic acid, via a decarboxylation.[2] It can act as either an attractant or a repellent for various insects. Commercially jasmone is used primarily in perfumes and cosmetics.
References
- L. Ruzicka; M. Pfeiffer (1933). "Über Jasminriechstoffe I. Die Konstitution des Jasmons". Helvetica Chimica Acta. 16: 1208–1214. doi:10.1002/hlca.193301601153.
- Paulina Dąbrowska, Wilhelm Boland "iso-OPDA: An Early Precursor of cis-Jasmone in Plants?" ChemBioChem 2007, Volume 8, pages 2281–2285. doi:10.1002/cbic.200700464
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.