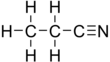
Propionitrile
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula CH3CH2CN. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds.[7]
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Propanenitrile[1] | |||
| Other names | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
773680 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.151 | ||
| EC Number |
| ||
| MeSH | propionitrile | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2404 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C3H5N | ||
| Molar mass | 55.080 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | sweetish, pleasant, ethereal[6] | ||
| Density | 772 mg mL−1 | ||
| Melting point | −100 to −86 °C; −148 to −123 °F; 173 to 187 K | ||
| Boiling point | 96 to 98 °C; 205 to 208 °F; 369 to 371 K | ||
Solubility in water |
11.9% (20 °C)[6] | ||
| log P | 0.176 | ||
| Vapor pressure | 270 μmol Pa−1 kg−1 | ||
Magnetic susceptibility (χ) |
-38.5·10−6 cm3/mol | ||
Refractive index (nD) |
1.366 | ||
| Thermochemistry | |||
Heat capacity (C) |
105.3 J K−1 mol−1 | ||
Std molar entropy (S |
189.33 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
15.5 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.94884–−1.94776 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H300, H310, H319, H332 | ||
GHS precautionary statements |
P210, P264, P280, P301+310, P302+350, P305+351+338 | ||
| NFPA 704 (fire diamond) | 
3
4
0 | ||
| Flash point | 6 °C (43 °F; 279 K) | ||
| Explosive limits | 3.1%-?[6] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
39 mg kg−1 (oral, rat) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[6] | ||
REL (Recommended) |
TWA 6 ppm (14 mg/m3)[6] | ||
IDLH (Immediate danger) |
N.D.[6] | ||
| Related compounds | |||
Related alkanenitriles |
|||
Related compounds |
DBNPA | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
The main industrial route to this nitrile is the hydrogenation of acrylonitrile. It is also prepared by the ammoxidation of propanol (propionaldehyde can also be used instead):[7]
- CH3CH2CH2OH + O2 + NH3 → CH3CH2CN + 3 H2O
Propionitrile is a byproduct of the electrodimerisation of acrylonitrile to adiponitrile.
In the laboratory propanenitrile can also be produced by the dehydration of propionamide, by catalytic reduction of acrylonitrile, or by distilling ethyl sulfate and potassium cyanide.
Applications
Propionitrile is a solvent similar to acetonitrile but with a slightly higher boiling point. It is a precursor to propylamines by hydrogenation. It is a C-3 building block in the preparation of the drug flopropione by the Houben-Hoesch reaction.
Safety
Propanenitrile is poisonous but weakly with an LD50 of 230 mg/kg (rats, oral).[7] Propanenitrile has been determined to be teratogenic due to the metabolic release of cyanide.[8]
In 1979, the Kalama (Vega) plant in Beaufort, South Carolina experienced an explosion during the production of propanenitrile by nickel-catalyzed reduction of acrylonitrile.[9] This site is now one of the two Superfund cleanup sites in South Carolina.[9]
References
- "propionitrile - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 6 June 2012.
- "Propionitrile". NIOSH Pocket Guide to Chemical Hazards. USA: Centers for Disease Control and Prevention. 4 April 2011. Identification. Retrieved 1 November 2013.
- Merck Index, 11th Edition, 7839
- CRC Handbook of Chemistry and Physics, 52nd Ed., p. D-153
- HSDB: Propionitrile, TOXNET, U.S. National Library of Medicine, retrieved 30 Oct 2015
- NIOSH Pocket Guide to Chemical Hazards. "#0530". National Institute for Occupational Safety and Health (NIOSH).
- Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_363
- Willhite, Calvin C.; Ferm, Vergil H.; Smith, Roger P. (1981). "Teratogenic effects of aliphatic nitriles". Teratology. 23 (3): 317–323. doi:10.1002/tera.1420230306. PMID 6266064.
- First Five-Year Review Report for Kalama Specialty Chemicals, Beaufort, Beaufort County, South Carolina, United States Environmental Protection Agency