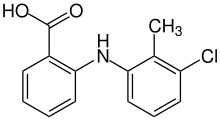
Tolfenamic acid
Tolfenamic acid (Clotam, Tufnil) is a member of the anthranilic acid derivatives (or fenamate) class of NSAID drugs discovered by scientists at Medica Pharmaceutical Company in Finland.[2] Like other members of the class, it is a COX inhibitor and prevents formation of prostaglandins.[3]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.862 |
| Chemical and physical data | |
| Formula | C14H12ClNO2 |
| Molar mass | 261.71 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It is used in the UK as a treatment for migraine.[4] It is generally not available in the US.[3] It is available in some Asian, Latin American and European countries as a generic drug for humans and for animals.[5]
References
- Andersen KV, Larsen S, Alhede B, Gelting N, Buchardt O (1989). "Characterization of two polymorphic forms of tolfenamic acid, N-(2-methyl-3-chlorophenyl)anthranilic acid: their crystal structures and relative stabilities". J. Chem. Soc., Perkin Trans. 2 (10): 1443–1447. doi:10.1039/P29890001443.
- Pentikäinen PJ, Neuvonen PJ, Backman C (1981). "Human pharmacokinetics of tolfenamic acid, a new anti-inflammatory agent". European Journal of Clinical Pharmacology. 19 (5): 359–65. doi:10.1007/bf00544587. PMID 7238564.
- NIH LiverTox Database Mefenamic Acid Last updated June 23, 2015. Page accessed July 3, 2015. Quote: "(fenamates generally not available in the United States, such as tolfenamic acid and flufenamic acid)"
- NHS Tolfenamic Acid (Tolfenamic acid 200mg tablets) Page accessed July 3, 2015
- Drugs.com Drugs.com international listings for tolfenamic acid Page accessed July 3, 2015
External links
- Tolfenamic acid information (Diseases Database)
Nonsteroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| Pyrazolones / Pyrazolidines |
|
| Salicylates |
|
| Acetic acid derivatives and related substances |
|
| Oxicams | |
| Propionic acid derivatives (profens) |
|
| N-Arylanthranilic acids (fenamates) |
|
| Coxibs |
|
| Other |
|
Items listed in bold indicate initially developed compounds of specific groups. #WHO-EM †Withdrawn drugs. ‡Veterinary use medications. | |
GABAA receptor positive modulators | |
|---|---|
| Alcohols |
|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates |
|
| Flavonoids | |
| Imidazoles |
|
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols | |
| Piperidinediones |
|
| Pyrazolopyridines |
|
| Quinazolinones |
|
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
Prostanoid signaling modulators | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
Ion channel modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium |
| ||||||||||||||||||||||||
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
See also: Receptor/signaling modulators • Transient receptor potential channel modulators | |||||||||||||||||||||||||